
Abstract of the battery tester
Best Battery Tester for Household Batteries. Batteries play an important role in everyday life, they provide the initial power needed to start the engines of cars to act as a backup source of electricity in telecommunications, public transportation and medical procedures. Many electrical devices use batteries as key components.
They are available in our vehicles, laptops, and CD players. A battery tester is an electronic device deliberated for testing the state of an electric battery, going from a simple device for testing the charge possibly present in the cells and/or its voltage output, its temperature, DOD, state of charge, state of health, to a more diverse testing of the battery’s condition, that is its capacity for collect charge and any possible flaws influence the battery’s staging and security.
Battery testers can do different measurements. Among the most common tests are actual battery capacity, battery cycle life testing, and characterization of battery dc internal resistance, (HPPC)Hybrid Pulse Power Characterization test (battery placed in in hybrids and EVs), (EDLC) electric double layer capacitor test, lithium-ion capacitor (LIC) tests, and several others.
Battery testers usually run capacity tests at the final battery charge and discharge rate to gain a more correct image of capacity than given by normal tests which take place at high charge and discharge rates.
Our aim is to make a battery tester that will be able to test 5v batteries as well as 12 volts batteries. And this system will test batteries with lithium-ion, hydrogen-nickel, Silicone, regular dry, plum-acid, and other types of battery. The system is designed in such a way that we get fast and efficient results and easily analyze the output.
Introduction of the battery tester
Background of the battery tester
Batteries are vital since they are power backup storage houses and cell phones. When there is no supply of electricity and power for future use, they supply energy. Battery applications are unable to count on fingertips. They are commonly used in manufacturing applications, residences, cars, cell phone applications, offices, laptops, and other medical devices.
First, what battery testing do we have to know? It is the procedure to verify if an Indian or foreign battery (EUT equipment under test or sample) conforms to and performs entirely. The battery tests provide a good picture of the three key parameters, power and self-discharge as well as internal resistance (means the ability to provide current). The charge amount must be retained for the proper functioning of the battery.
Batteries are very expensive to purchase, and battery life is advertised as 9-10 years, but typical battery life must be replaced after 5-6 years. The battery test shows us the battery’s clear picture. Generally, when the safety requirements are passed and all the test formalities pass, batteries are sold out at a higher price. Testing the battery results in many parameters such as battery life and capacity.
In compliance with IS 15549, IS 5154, IEC 61427, IEC 60896-21, IEC 60896-22 IEC60896-11, ITC India Pvt has the capacity for testing the Lead Acid batteries. It is a NABL certified laboratory to conduct tests for lead acid batteries, acceptance tests and efficiency tests.
Since in this modern age batteries take an hour, we have the right to buy a genuine and healthy product. For our economy and protection, battery testing is therefore important.
The ITC Life Cycle Tester is a laboratory equipment developed for battery life testing.
Our Aim of make a battery tester
Our aim is to make a device that will be able to test 5 volts and 12 volts battery and do certain type of test, to prevent our battery from major damage and know the condition of the battery before wasting.
Objectives of the battery tester
The aims of the study are thus:
- Create a low-cost battery tester.
- Develop a tester that shows the current, voltage and capacity of the battery attached.
- To test lithium-ion batteries as well as lead acid batteries.
- To display real time values on LCD screen.
- The project aims to improve performance, lifetime, and total cost of batteries.
- Standardized methodology for battery life testing.
- Methodology for lifetime prediction.
- Measuring state of health and state of charge of the battery
Scope of the battery tester
The scope of this project is to show the actual condition of the battery, either 5V battery or 12V battery. It can be used in cars, emergency power, communication base stations, robots and so many other places. It can be used everywhere, where batteries are installed.
Motivation for battery tester
There are many batteries testers on the market, but they are available with limited testing. Some of these testers can test voltage and some can test current. These testers cannot show us the actual condition of our battery and our battery becomes waste, because sometime the battery is overcharged or fully discharged or turns dead.
To solve this problem, we will bring a solution, we will make a device which will be able to do different types of tests, which will show us the actual condition of our battery and thus we can protect our battery from major damage.
Problem Statement according to a battery
A successful diagnostic, preventive maintenance and corrective service program encompassing the life of a battery is essential to a quality maintenance program. The diagnostic section checks the condition of the battery.
Condition monitoring helps avoid or reduce premature battery loss and overturns or corrects a problem that has already arisen. Ensuring maximum efficiency in the electrical system of the cars, reducing maintenance costs associated with batteries, removing jump starting, extending battery life, meeting environmental targets, and reducing the number of new batteries purchased.
In general battery protection should address the following undesirable events or condition
⦁ High self-discharge.
⦁ Excessive current during charging or discharging.
⦁ Short circuit.
⦁ Over voltage – over charging.
⦁ Under voltage – exceeding present depth of discharge (DOD) limits
⦁ High ambient temperature
⦁ Over heating – exceeding the cell temperature limits.
⦁ Pressure built up inside the cell.
⦁ System isolation in case of an accident abuse
⦁ Abuse
Literature Review for the battery tester
This system will test batteries with lithium-ion, hydrogen-nickel, Silicone, regular dry, plum-acid, and other battery types. Characterization of hybrid pumps and hybrid (HPPC) tests, double-level electrical condenser (EDLC) testing, lithium-ion condenser (LIC) testing, and other tests. It can be broken down into core test tester, completed battery tester, battery tester for cell phones, battery tester for notebooks and battery tester for mobile DVD. The battery tester will perform thorough testing results.
Many electrical devices use batteries as key components. They are available in our vehicles, laptops, and CD players. A battery is simply a can full of electron-generating chemicals. There are two terminals in the basic battery structure. One is positive, the other is negative. The terminals are the ends of the battery of regular flashlight batteries. Two heavy plumbing posts serve as terminals in a large car battery.
On the negative battery terminal, electrons accumulate. The electrons flow as rapidly as possible from the negative to the positive terminal when connected between the negative and the positive terminals. Use the wire to connect those forms of load to the battery. The load may be like a lighthouse, engine, or electronic circuit such as a radio.
The electrons are produced in the battery itself by a chemical reaction. By this chemical reaction (internal battery resistance), the speed of development of electrons influences the number of electrons between terminals.
Electrons flow through a wire from the battery and must pass from the negative to the positive terminal to produce a chemical reaction. For a year a battery may therefore sit on a rack and still be strong.
In 1800, Alessandro Volta had traditionally produced the first battery. He created a battery by alternating zinc layers, bloating paper soaked in salt water, and silver to create his battery. It was called a voltaic pile in this arrangement.
The pile must contain various metals on the top and bottom layers. The voltage and current from the stack can be measured by connecting a wire to the top and bottom of the stack. The battery is stackable as high as possible, and the voltage is increased by a fixed voltage each layer. A Zinc/carbon battery is the simplest battery that can be made.
A zinc rod is placed in a jar full of sulfuric acid. The acid starts to eat at zinc instantly. The zinc rod will create hydrogen gas bubble, and the rod and acid start to heat up. Many reactions are occurring. The acid doesn’t matter when a carbon rod is put into the acid. You can use the electrons which flow through the wire and measure the voltage and current in the wire to power a light bulb or similar load. Any heat energy is converted into electron movement. The electrons find it easier to merge with hydrogen to pass through the carbon bar.
Finally, the zinc rod is completely dissolved, or the acid hydrogen ions are exhausted, and the battery dies. Modern batteries use a range of chemicals to react. The following are some of the battery chemicals. There are zinc/carbon batteries, as mentioned previously. They’re also considered to act as regular carbon batteries, and all cheap AA, C and D drizzle batteries have zinc/carbon chemistry. The electrodes are zinc and carbon with an electrolyte paste between them.
The secondary type of battery is the oxidizing battery used in common batteries Duracell and Energizer. Electrodes with alkaline electrolyte in this form of pile are zinc and manganese-oxide. [7] The lead-acid battery used in automobiles is another popular battery. Bley and plum oxide with a solid (rechargeable) acid electromagnetic oxide are the electrodes.
Multiple batteries are used simultaneously on virtually any computer that uses batteries. The batteries are collected in serial or in parallel to the creation of higher voltages. The voltages add up in a serial environment. The currents are added in a parallel arrangement.
When every cell produces 2 volts in a parallel arrangement, four batteries produce 2 volts in parallel. The present will be four times that of one cell, however. The voltage of 4 outputs is 8 volts in serial mode.
Essentials in Battery Testing
There is no realistic method in a fast, thorough test for the quantification of all battery conditions. Unable to calculate state of health (SOH) per se, the precision can only be measured at different levels based on the available symptoms. An accurate calculation is not possible if the signs are ambiguous or uncertain. Three SOH measures must be measured when evaluating a battery:
- Capacity and energy-saving
- Internal power, the capacity to supply current, and
- Discharge that reflects mechanical integrity and conditions of stress
In certain conditions the battery occurs, and a charge can easily hide a symptom that can work well in a poor battery. A powerful low-charge battery also has similarities to a pack that can lose power. Also, recent loading, discharge, or long storage affects battery characteristics. When checking batteries, these mood shifts must be clearly established.
The battery’s primary health metric is capacity, an energy storage calculation. 100% of the rated capacity can be provided by a new battery. A 5Ah pack is expected to supply five amps for 1 hour. When the battery stops in 30 minutes, the power is 50%. Capacity also facilitates the substitution of guaranteed obligations that fall below 80%. Over everything the capacity determines battery life’s end.
Lead acid starts at about 85% and rises in capability until the long and steady decline begins. Lithium ion starts on top and immediately, but very slowly begins declining. In the event of new or post-storage nickel batteries, they must be primed to achieve maximum performance.
The device criteria for a new battery are dependent on manufacturers. This state is transient and in real-life circumstances is not a battery, as it starts to fad from the day it takes place. The decrease in performance only comes to be noticeable once the shine of a new device has worn off and daily routines are being taken for granted.
Problems Faced by Battery User
It is very difficult for many batteries users to answer someone’s question when he asks him how you replace your battery or at what capacity you replace your battery, and he confusedly response that I forgot. There are few who know the term capacity, but capacity is a very important term in battery, it is used as a battery removal threshold, and it is a runtime metric.
The retirement of the battery depends on the use. The replacement threshold is normally set at 80% by organizations using battery analyzer. Some companies will keep the battery longer than others and there is a toss between the ‘what if’ and the economies. In stores, scanning machines can go as low as 60% and can still work a full day. A starting battery in a car still dials well at 40%, but it’s small.
Any mission powered by battery needs to prepare a worst-case scenario. While producers have reserves to determine runtime, the quantity is rarely explicit. Critical missions require tolerances that are tightened, and the battery must be replaced before a sudden loss can be tolerated.
Essential and sometimes over-replaced batteries are medical and military equipment. Installation makers tend to use a cycle count or date stamp to order withdrawal, instead of checking them. The service life on a date stamp is frequently restricted to 2 or 3 years to cover all eventualities.
Medical technicians find that many of the defibrillator batteries have a power of more than 90% before a 2-year compulsory date-stamp expires, which replaces excellent medical batteries prematurely.
Despite this obvious waste, an American FDA investigation says that “up to 50% of service calls in hospitals surveyed have to do with battery problems “AAMI (Association for Improving Medical Instruments) health professionals also state that “battery management was the top 10 medical system challenge.”
Another battery capability application in a drone is significant. The unit will fly for 60 minutes using a good battery, but if the power is decreased from 100 percent to 75 percent, the flying cost is short to 45 minutes if the mission control is unknown.
The $25,000 vehicle could crash when a second landing approach is needed to negotiate. Battery batteries with a capacity of nearly 100 percent which be allocated for long distances while batteries for shorter transactions are marked as part of battery maintenance. This enables every battery to be used to its fullest and ensures a strong pension policy.
A fuel gauge is indicated by the remaining energy in many batteries and portable devices. If the battery is fresh or faded, the full charge still shows 100 percent. The prediction that a battery with full charge can deliver the same runtime as a new one provides a false sense of protection. Only SOC and not power is indicated by batteries of fuel gauge.
Not only handheld devices are the battery failure restricted. Starter batteries are also vulnerable to failure in cars. In 2008, ADAC said that 40% of all roadside failures had to do with battery systems. A 2013 ADAC study says that between 1996 and 2010, battery issues quadrupled.
ADAC, the biggest auto club in Europe, also reports that a battery discharged or faulty includes any third breakdown. The study was released in May 2013 by German Motor Welt, and states that the average age of five years is just a few starter batteries. All cars are subject to this.
The figures were based on the four million accidents usually occurring in one year at the ADAC car club. The analysis included only new vehicles; vehicles over 6 years old were not qualified for service.
Similar findings are reported by BCI (Battery Council International). In 2010, a BCI scientific subcommittee report found a 9% rise from the previous five years in grid-related failures. Experts believe that higher electronic demands contribute to higher failure rates in modern vehicles.
The biggest single complaint amongst new car owners is battery failure in Japan. The medium-sized car drives 13 km/day and in overcrowded towns mostly. Battery failure is triggered most often by the under load, which causes sulfating. The performance of the battery is essential; problems are reported as component failure and customer satisfaction during the warranting period.
A German luxury car manufacturer announced that there were no trouble returning one in two starter batteries under the guarantee. A high-quality starter battery German manufacturer claimed that factory defects constitute only 5% to 7% of all warranty claims. During the guarantee period, battery failure is rarely a factory error.
Key culprits are driving habits. An in-depth evaluation of different failure symptoms using advanced battery test instruments will substantially reduce warranty requirements.
Related battery warranty problems exist in the smartphone industry. It is said that nine of the 10 batteries returned have no problems. The employee supersedes the battery rather than manipulate a consumer complaint due to less than planned runtime. This stresses the vendor and can lead to repeated complaints without fixing the issue.
What are the Classification of Batteries
Batteries can be classified based on charging and discharging ability. They are then further classified based on designs and electrolyte used.
What are the Primary Batteries
These cells cannot be recharged electrically easily or reliably and therefore, until discharged and refused. Many of the primary electrolyte cells are a material that is absorbent or separator (no free, or liquid electrolyte) is included So-called ‘dry cells.’
The main battery is not very heavy, typically cost-efficient packaging source. Mobile electronics and electrical equipment control, lighting, photography equipment, Toys, backup memory and a host of other apps that offer free use Power.
Main batteries have overall advantages of good shelf life and high energy Density, maintenance, and ease of use at low to moderate release rates. While large primary batteries of high capacity are used in military applications, the overwhelming majority of primary batteries are signaling, standby control, and so on popular single cell batteries with flat and cylindrical button.
What are the Secondary Batteries
These batteries can be recharged in their original conditions electrically after discharge by moving current to the discharge current through them in the opposite direction. They are electrical storage devices and are also known as “storage batteries” or “Batteries.” Secondary battery applications fell into two major categories.
Apartments used as a holding capacity for the secondary battery, are normally attached to the main energy supply and electrically charged.
1-The energy supply on demand for the load. Examples include automobiles and aviation’s Energy, Hybrid and Pure Electric Systems, No-fail, and Standby Emergency (UPS) Electrical power load leveling systems for automobiles and stationary energy storage systems (SES).
2- Applications in which the secondary battery is basically used or discharged Main battery but recharged instead of discarded after use.
Secondary batteries are used like this in portable consumer electronics, power tools, for example, for cost reduction, hybrid cars, etc. (secondary batteries are recharged rather than replaced),
and in applications that need power beyond primary battery capacity High power density, high rate of discharge, flat battery characteristics Curves for discharge and good low performance temperature. The densities of their energy are less than the main batteries usually.
Certain batteries are recharged” by “mechanically charged types” Replacement, typically with a fresh metal anode of the discharged or depleted electrode One. Some of these types of batteries are defined by metal/air batteries.
What are Reserve batteries
A main component is isolated in these primary forms from the rest of the battery before Enabled. Chemical degradation or auto discharge in this state is basically removed and long-term battery storage is capable.
The electrolyte is usually the isolated part. The battery is present in different devices including the thermal battery Inactive until a solid electrolyte is heated, and then conductive. The battery reserve architecture can be used for very long or harsh climates.
Storage requirements that cannot be fulfilled with the same “active” battery Characteristics of success. For example, these can be utilized to provide supply high power for relatively short time periods such as missiles and other weapons.
Fuel Cells use as a battery
Fuel cells are electrochemical galvanic cells, like batteries, that change chemicals directly into electricity and not subject to the thermal constraints of the Carnot cycle Motors. Motors. Battery-like fuel cells are but active materials are an integral component. A system component (as in a battery) is fed from an integral source into the fuel cell if it is desired force.
The cells of fuel vary in their power from a battery Electrical energy generation if the active material is fed to the electrodes there was a mistake (assuming the electrodes do not fail). The energy generated by the battery stops when the battery’s limiting reactant is consumed. The fuel cell’s electrode content is inert since it is not eaten but has properties (catalytic) that improve electro-reduction or reactants’ electro-oxidation (the active materials).
In fuel cells, the active anode materials usually are in gas or liquid form having metallic anodes typically used in majority of batteries) and fed into the side of the anode the cell of the petrol. Since these materials mimic more traditional heat engine fuels, in describing these devices, the word “fuel cell” is now common.
The air or oxygen, the prevailing oxidant, is supplied to the fuel cell side of the cathode. Fuel cells have been theoretically more powerful and less necessary for more than 150 years. Conversion to electricity of hydrogen and carbon or fossil fuels Compared with traditional motors. A well-known fuel cell application was in space vehicles for more than 40 years, hydrogen/oxygen fuel cell is used.
Fuel use Cells have grown slowly in terrestrial applications, but recent innovations have revitalized air respiration systems interest in a wide range of applications like utilities Strength, leveling load, scattered or on-site electric generators and electric vehicles.
Advantages of Battery Testing
Battery tests are performed to estimate health status, cycle life, real ability, battery, or bank charge status. Two methods for battery testing are available, invasive, and non-invasive. These methods detect the facts of the battery but do not explain why. Here are several standard battery testing methods:
What is a Methods invasive
- Determination of electrolyte density: the state of health is calculated. This does not refer to VRLAB
- Electrode Tests Potential: Depletion of Positive active weight and negative active weight.
What is a Non- Invasive Methods
- Float voltage and open circuit: Health status and charge status are calculated.
- Monitoring battery/cell temperature: Health status, shorts
- Measurement of Charge/Discharge voltage: health condition, charging status is determined.
- New Download: Capacity determination of the actual battery.
What is Unload test of a battery
One of the most critical tests of battery tests is unload testing. This is the best way to demonstrate the battery or battery line’s true power (battery or bank indicates whether the required voltage is supplied by two or more batteries and cells). The results are accurate, but time-consuming and costly.
For discharge testing, battery testing and selective discharge testing may take place. Battery checks may take place. The India Pvt Ltd Research and Certification Institute has a lithium-based battery life-cycle test.
It performs control tests on the car battery. The discharge phase of the life cycle measuring unit is constant with air-cooled transistors. The greatest versatility for loading is in loading and unloading LCT systems. These circuits are designed to prevent shortcuts and large fluctuations in input power.
⦁ Visual inspection: As its name suggests, it is important to check for cracks, corrodes or leaks without visual testing. It does not notify us of the battery power, condition or loading status of the string or of a single battery.
⦁ Impedance testing: This test cannot determine the state of health of the battery. Impedance monitoring shall be carried out on a quarterly basis. Over time, the battery life is decreasing in several ways and this testing is critical for checking the battery’s health. It is just a 35-minute exam and not one that takes time.
What is Capacity Testing of the battery
Capacity is a battery’s key health measure, but it is difficult to estimate on the fly. The conventional charge/unload/load cycle is still the most accurate way of calculating battery power. A complete cycle on a big plum lead acid battery is not feasible for total capacity calculation though remote batteries can be used again reasonably quickly.
Different method of capacity testing
Non-intensive method of capacity testing
(EIS) electrochemical impedance spectroscopy multimodal that looks over the protection of battery by scanning, is used by Spectrum. Invasive technology integrates EIS with complex capability evaluation modelling, CCA and SOC, using the matrixes often referred to as search tables.
A multiple frequency sinusoidal signal is pumped with several millivolts into the battery. The resultant signals give a Nyquist plot after digital filtering, which is superimposed by different electro-chemical prototypes. Spectrum chooses the best model to fit; replicas that are not fitted are rejected. The fusion of data then correlates main parameter values to capability and CCA estimates. Figure 2 demonstrates in a condensed way the proprietary mechanism.
Discharge method of capacity testing
To calculate the power of a battery it takes a resistor or some other charge to discharge the batteries until the voltage drops to a minimum value and during the discharge process, the current and voltage are registered. Then construct a graph with the data gathered and the battery capacity from this data can be estimated. There is one problem here though, that during unloading we must integrate the data over time so that the current across the load resistor falls down.
However, if we discharge the battery through a source, we can easily and more accurately calculate the battery’s strength. The other problem, however, is the tension of the entire battery (1.2…3.7 V) is not adequate for control. A further voltage source can be used to solve this problem.
How to measure the capacity of a battery
First the value of the Resistor R1 corresponds to the desired output current. The discharge current in most situations equals the current of the battery. Notice that some LM7805 and others voltage regulators will consume 2…8 mA of extra current, so it is best to verify the current by an ammeter.
Turn on the SA1 switch and remember the time afterwards, connect the fully charged battery to a circuit board. Look at the reader’s voltmeter (PV1). Switch off the SA1 switch and remember it again when the voltage of the battery has a minimum value. Recall that deep download can shorten or damage battery life! [15]
The battery capacity performance (in Amperes per hour) can be estimated by multiplying the discharge current (in amperes) by discharge time (in hours):
C = I * t
⦁ For various battery types, the minimum battery voltage varies. For example, for a battery with nickel-cadmium (NiCad), a minimum voltage of 1.0 V is required, for a battery with Nickel-metal hydroid (NiMH) –1.1 V, for a lithium battery with a lithium-ion Li-ion), -2.5….3.0 V…
What is State of Charge (SOC) of the battery
The charging condition is very important for batteries, but the concept of it poses several different challenges, typically the charging status id is determined by its actual power ration ((t)) to the nominal capacity (Qn). The manufacturer’s nominal capacity is given, and the full charge can be stored in the battery. The definition of SOC may be as follows: SOC (t)
Q(t)> present capabilities
Q(n)> Capability nominal
Various state of charge mathematical system
⦁ Direct measuring: physical battery characteristics such as battery tension and impedance are used for this process. Bookkeeping estimates the current discharge method is used as the source and the download current is integrated over time to measure SOC.
⦁ Adaptive systems: the adaptive systems are self-designed, and the SOC can be modified to multiple discharge situations automatically. Different new adaptive mechanisms have been developed for the SOC estimate.
⦁ Hybrid approaches: hybrid models benefit from the benefits of each SOC estimate protocol and give an optimum estimate efficiency on a global basis. The literature indicates that hybrid methods typically generate a successful SOC evaluation in contrast to single methods.
Direct calculation statistical methods Process of open circuit stress.
(ii) Form of terminal voltage
(iii) Method of change
⦁ Spectroscopy impedance technique
Book-keeping forecast statistical methods
I Method of Coulomb Counting
(ii) Method of counting modified coulomb
Adaptive processes mathematical approaches the BP Network for Nerves
(ii) The RBF Network for Nerves
(iii) Vector machine support
(iv) Network of Fuzzy Nerves.
⦁ Filter Kalman
Mathematical methods of Hybrid methods
⦁ EMF mixture and coulomb counting
⦁ Mixture of coulomb counting and filter Kalman
⦁ EKF mixture per unit device
Internal Resistance Testing of the battery
DC load method of internal resistance testing
⦁ One of the common and most accurate research techniques is ohmic calculation.
⦁ A brief second or longer discharge is given to the battery.
⦁ The load current is 1A or less for a small battery; it may be 50A or more for a starter battery.
⦁ Open circuit voltage (OCV) testing the voltmeter without charge •
⦁ Take loading second reading
⦁ Laws of Ohm measure the value of the resistance
DC Load Messing’s are ideal for testing big, fixed batteries and the device’s Ohmic readings are very reliable and reproducible. High-end test instruments in the 10 micro-ohm region claim resistance readings
The DC load system of two levels provides an alternative method by adding two consecutive loads of varying currents and periods. The initial dislodging of the battery takes 10 seconds at low current followed by a higher current for 3 seconds (see Fig. 4). A battery measurement of the voltage signature under two loading conditions does provide additional details, but the values do not disclose SOC or power estimates. SOC is purely resistive. The load test for batteries that charge DC is the preferred method. [20]
AC CONDUCTANCE method of internal resistance testing
⦁ In 1995 Keith Champlin first mentioned it.
⦁ A linear association between load and conductance testing is developed.
⦁ Capacitate and inductive reactance combine with the lead acid batteries 70-90Ah in injecting a frequency of roughly 90 Hz to a marginal voltage latency that minimizes reaction. (Smaller batteries increase this level and a wide battery drop.)
⦁ AC driving meters are used for CCA calculation in car garages.
One more popular form is the 1,000-hertz (Hz) ohm measure. A signal of 1,000Hz excites the battery and the law of Ohm determines its resistance. Note that when calculating a reactive resistance, the AC method displays different values to the DC method and both readings are valid.
Li-ion generates approximately 36mOhm in 18650 cells with a signal of 1,000Hz AC and approximately 110 m ohm for a DC load. The user must accept the submission since both readings are accurate but far apart. The pulse DC loading method provides precious readings for a DC application such as a heating system or a light, whereas the 1000Hz method better represents the performance needs of the digital load, such as mobile computing and mobile telephone technology, which largely relies on the battery capacities. [21]
Electrochemical Impedance Spectroscopy (EIS) for internal resistance testing
For several years, testing laboratories have used EIS to test the characteristics of the battery. The technology is restricted to laboratory settings with high cost of facilities, slow test times and the need for qualified experts to decode vast quantities of data. However, EIS reads R1, R2 and C values in the Randel model, which compares the data with the CCA and involves complex modelling. [21]
What are the operations of a battery?
What is Mean by Discharging of a Battery
Figure demonstrates the activity of the cell during discharge. If so, the battery is attached to a load (external) and the anode electrons flow. Oxidized by the external load to the cathode, which accepts electron the content of the cathode is decreased. In the electrolyte the electric circuit is completed by Anion flow (negative ions) to the anode and cathode and cation flow (positive ions), Each of them.
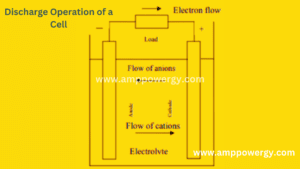
Discharging can be shown as the following reaction, with assuming metal and chlorine (Ci2) as anode and cathode, respectively.
Negative electrode: anodic reaction (oxidation or loss of electrons)
Zn → Zn2+ + 2e
Positive electrode: cathodic reaction (reduction or gain of electrons)
Cl2 + 2e → 2Cl-
Overall reaction (discharge):
Zn + Cl2 → Zn2+ + 2Cl-
(ZnCL2)
What is Mean by Charging a battery
When a rechargeable or store cell is filled, the current flow is reversed. The positive electrode is oxidized and as seen in figures; the negative electrode is reduced. Show. As the anode is, by definition and cathode, the electrode where oxidation is performed, the positive electrode, where the anode is diminished, and the cathode is negative.
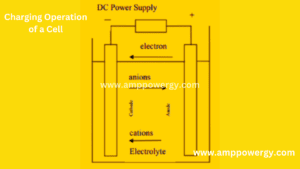
In the example of the Zn/Cl2 cell, the reaction on charge particle can be written as follows:
Negative electrode: cathodic reaction (reduction or gain of electrons)
Zn2+ + 2e → Zn
Positive electrode: anodic reaction (oxidation or loss of electrons)
2Cl – → Cl2 + 2e
Overall reaction (charge):
Zn2+ + 2Cl-→ Zn + Cl2
Factors effecting battery health.
Operational features, power, energy production is subject to several factors and a battery’s output. The battery output impact of these factors This segment has been discussed. It is worth noting that many things are possible.
These results can only be viewed as generalizations and the effects in more strict operating environments, each factor is typically larger. For the first time. For example, not only high storage effects are more marked Temperature and long periods of storage but also in harsher environments After stock, discharge.
The observed loss after a certain storage time Capacity is typically larger under heavy discharge (compared to a new battery) Loads under luminous loads of discharge. The observed power loss at low temperatures is comparable (comparable to normal).[21][20] Discharge temperatures) would be higher at heavy than moderate or light discharge Loads.
It is also recognized that there will be output even in a specific cell or battery design Differences between manufacturer and manufacturer and various variants of the Battery the same (such as standard, heavy-duty, or premium). Performance is also available variables inside a production lot and within a production lot and a production lot Any development process inherent. Depends on the magnitude of the variability Controls of processes as well as battery application and usage. Produces data Relevant output features should be consulted.
Battery Tester Project components and software
As the name tells us, in this chapter we will discuss the software and hardware used in this project. In Sofware section, we use Arduino uno as a microcontroller and they are briefly explained as given below, now in hardware section briefly explanation of different components used in this project which include Pin configuration of OLED, sensor, buzzer, mosfet.
Hardware Used in Battery tester
The hardware used during the venture and our intention of using it are described below.
Microcontroller used in Battery tester Project
We use Arduino Uno having Microchip ATmega328P.
Overview of the Microcontroller used in battery tester
“Arduino Uno is a microcontroller open source. Arduino Uno was developed on ATmega328P microchip Arduino. It has optical and analog I/O pins, which can be attached to different shields and other circuits. The board is made up of 14 I/O digital pins and six analog I/O pins. The board consists of six pins. The boards often come with a USB cable or external 9-volt battery, but they have a voltage of between 7 and 20 volts. And so are Arduino Nano and Leonardo.”
Memory of the microcontroller used in project
The development board has 256 Kilobytes of flash storage for encrypting code (including 8 kilobytes also for compiler), 8 KB of Main memory, and 4 KB of Flash memory (including the EEPROM library for reading and writing).
Programming of the microcontroller of the project
Using Arduino (download) software to program the Arduino microcontroller. The Arduino ATmega2560 microcontroller is pre-born with a bootloader that provides users with access to programming without having to use an external hardware programmer. It interacts with the original protocol of STK500 (reference, header files in C). The boot disk can also be circumvented, and the processor set up via the ICSP header.
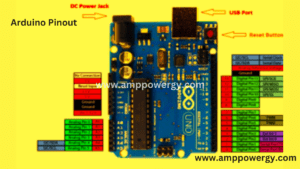
Communication Processes in the microcontroller
“There are a variety of digital and analog I/O pins on the 5V frame. These pins are fitted with regular ratings from 20mA to 40mA. The board employs internal pull-up resistors limiting the current that exceeds the operating conditions.
But too much current increase means that these resistors are unprofitable causing hazard to the system. Arduino Uno comes with an integrated LED linked with pin 13. The HIGH value of the pin turns it on, LOW turns it off. Wine. Wine. This is the Arduino Board’s input voltage. The USB port offers different than 5 V. The voltage is supplied with this pin. If a voltage is supplied via a power jack, this pin.5V is available. This board can control the voltage. Using a 5V pin, controlled voltage is given output.
The board is controlled by three processes i.e., USB, DC power jack or Vin board pin. USB supports 5V voltage while Vin and Power Jack support the 7V to 20V voltage range. The board should be run on 5V. It’s vital to know that when voltage is given via 5V or 3.3V pins, it bypasses the voltage control that can harm the board when its voltage reaches its limit. It is important to note that GND, for example. There are pins on the field. On the board, more than one ground pin can be added, as necessary. Reset. Restart. This pin is included in the board that resets the onboard software.
The IDE is supplied with a feature that resets the board through programming instead of a physical reset on the board. OREF. OREF. This pin is very useful to refer to the board’s voltage. The voltage across this pin is read by a shield, which then selects the correct power source. PSM. PSM. 3,5,6,9,10, 11pins PWM is delivered.
The 8-bit output PWM.SPI is available for these pins. The serial peripheral interface is known. The SPI contact with the aid of the SPI library includes five pins 10(SS), 11(MOSI), 12(MISO), 13(SCK). AREF. AREF. Analog comparison is named. This pin gives a reference voltage for the analog inputs. The second. The second. Two wire interfaces are named. The Wire Library is available for TWI connectivity.
Pins A4 and A5 are therefore included. Therefore. Serial correspondence. Communication. Serial correspondence. Communication. Serial contact is made between pins known as pin 0 (Rx) and pin 1 (Tx). The pin Rx (0) is used to retrieve data while the pin Tx (1) is being used to relay data. Breaks outside. Pins 2 and 3 are used for outside interruptions. If the value is low or changed, an interrupt is called.
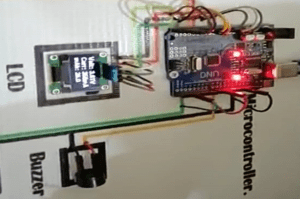
OLED Display used in battery tester
Overview of the OLED Display
“OLED is a unique direction light emitting device that uses a series of organic thin films between two wires. The technology is flat lighting emitting technology. A bright light is produced when electric current is applied. OLEDs are emitting displays with no backlight and thus are thinner and more powerful than LCDs (with a white backlight).
There are two primary OLED families: small molecules and polymer families. Adding mobile ions to an OLED produces an electrical cell that emits light (LEC) and is operated in a very different way. A passive (PMOLED) or active (AMOLED) controller may be used to operate an OLED display. Under the PMOLED scheme, every line (and row) is sequentially controlled, 1 by 1 [11], while the AMOLED controller uses a Thin Film Transistor backplane to reach and turn every single pixel on and off so that higher resolution, bigger display sizes are available.
What is the Features of the OLED Display
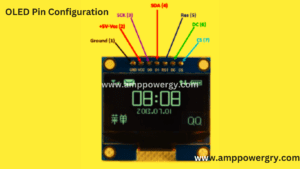
- Wide range of voltages: 3.3V-5V DC.
- Angle of viewing: over 160 degrees
- SSD1306 Driver IC:
- Tailor: 1.25″
- Notice: IIC, 2 I/O pins only!
Important Applications of the OLED display
Televisions
- Displays for cell phones
- Indicators of the machine
- Taste Panels
- Lights
- Screens for handheld computers
What is MOSFET (IRFZ44n)
Overview of MOSFET (IRFZ44n)
The IRFZ44N is a MOSFET N-channel with a 49A, low-wheel drain. 17.5 m worth of boy. The MOSFET is also powered by a low threshold voltage of 4 V. It is therefore typically used for 5V driving by microcontrollers. If the MOSFET must be switched entirely, a driver circuit is required.
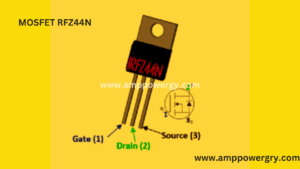
- Specifications
- Small signal for N-channel MOSFET
- The present drain (ID) is 49A at 25oC.
- The new pulsed drain (ID) has a current of 160A.
- The minimum door’s threshold stress (VGS-th) is 2V
- The gate threshold’s highest voltage is 4V (VGS-th).
- The gate-source voltage (VGS) shall be ± 20V (max);
- 55V gross stress drain in short form (VDS) • Drain root gross stress.
- It’s about 60ns and 45ns to go up and down.
- The low threshold current used by Arduino.
- The kit to-220 Introduced
How to Use MOSFET (IRFZ44n)
In comparison to MOSFET transistors, the devices are tension regulated. This means that the appropriate Gate threshold voltage (VGS) can be switched on or off. IRFZ44N is a MOSFET N-channel so when zero voltage is applied to a gate pin drain and source pins will be open. These pins are closed when a door voltage is applied.
If you must turn to Arduino, a simple transistor drive circuit works to supply the necessary voltage for triggering a MOSFET completely open. A dedicated MOFET Driver IC is needed for other switching and amplification applications.
IFRZ44N with 5V gate
If the MOSFET Gate Pin is joined directly with the I/O pin of an Arduino, PIC, etc. The drainage current does not fully open and relies on the voltage applied to the gate pin. The graph below shows how much drainage current is allowed for the 4V to 10V gate level voltage.
- Applications
- Switching of high stress devices
- Pace checking of the motor
- Dimmer or LED
- High performance switching systems •
- Adapter or inverter circuitry
Role of the Resistor in battery tester
A resistor is a two-terminal passive electric component which uses the electric resistance part of the circuit. Resistive is used in electrical circuits, including in applications to reduce current flow, adjusting signal levels, voltage dividing, active bias and terminating transmission lines.
In the distribution systems, high-power resistors can also be used as test loads for generators that can decrease electric power by many watts, as heat. Set resistors are only slightly shifting resistors, including temperature, time and stress. Circuit elements (such as volume control or dimmer) or sensor systems may be adjusted with variable resistor elements for heat, illumination, humidity, strength, or chemical activity.
Role of the Buzzer in battery tester
Mechanical, electromechanical, or dielectric audio signaling devices (short piezo) are buzzers or beepers. Alarms, timers, and user input validation such as a mouse click, or a keystroke include common use of buzzers and beepers.
- Specifications
- Ratio of voltage: 6V DC.
- Organizational stress: 4-8V DC
- Approval: <30mA
- Type of tone • Constant beep •
- Rate of resonance: around 2300 Hz.
- Slim, flat enclosed packaging
- Pleasant board and great breadboard
How to use a Buzzer in battery tester
A buzzer is a small but powerful part of our projects/systems to add sound features. It is a very small and compact 2-pin structure which makes this widely used in most electronic applications simple to use on boards, Perf boards and even on PCBs.
Two styles are often available buzzers. The one here shown is a simple buzzer that makes a Persistent Beeeeeeppp when powered…. Tone, the other type is referred to as a ready-made buzzer that looks bulkier and produces beep sound. Sound because of the internal circuit inside it. However, the one shown here is most used because it can be easily adapted to our application using other circuits.
This buzzer will be powered by the 4V up to 9V direct current power supply. A basic 9V battery can also be used, but it is better to have a +5V or +6V DC power operated. The buzzer is normally combined with an interval to trigger or disable the buzzers with an interval.
Important Applications of the Buzzer
- Circuits that warn the consumer about something
- Equipment of correspondence
- Technology for cars
- Handheld smartphones, owing to their small size.
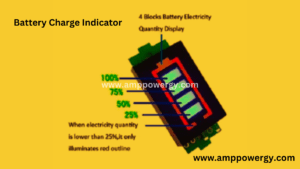
- 1s Battery Charge Indicator
1s Battery Charge Indicator
The IS battery capacity sensor can be used as a basic visual guide to the recharge status of 1 650 Lithium-Ion Batteries and Certain Lithium Batteries at 4.2 volts per cell maximum voltage to provide short, easy to read battery voltage condition for DIY, battery chargers and RC and electric cars. The display is red, and the bar is seen with the blue lithium battery power.
What is a Temperature Sensor (Digital)
The KY-028 is a temperature sensor (digital) for Arduino tests temperature variations depending on the resistance of the thermistor. There is a potentiometer for changing the detection rate on the digital interface, both digital and analog.
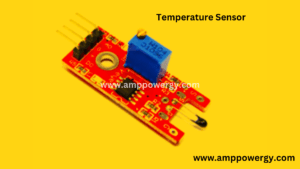
Temperature Sensor
Connections of the temperature sensor
Link the analog output of the board (A0) to pin A0 and digital output (D0) to pin 3. Link 5V and GND terminal (+) to the ground (G).
KY-028 Arduino
A0 A0
G GND
+ 5V
D0 2
The digital interface sends a HIGH signal on the Arduino LED (pin 13), when the temperature threshold is reached. To raise the detection threshold and counterclockwise to lower it, transform the potentiometer clockwise.
The analog interfaces return a numerical value which depends on the location of the potentiometer and the environment. As we did with the analog power output pin KY-013 directly connected to the resistor, the Steinhart Hart formula cannot be used to calculate temperature, this factor can be used to measure relative temperature difference.
Specifications of the temperature sensor
The KY-028 which is consists of a thermistor of type NTC, a two dissimilar LM393 comparator, a potentiometer of trimmer of a type 3296W, six electrical resistors and two LEDs (light emitting diode) of the predictor. The board has a digital and analog output.
Operating Voltage 3.3V to 5.5V
Temperature measurement range (TMR) -55°C to 125°C [-67°F to 257°F]
Measurement Accuracy ±0.5°C
Board Dimensions (BD) 15mm x 36mm [0.6in x 1.4in]
What is a Battery Capacity Tester
Resistive loading can severely burn, pay attention to safety thanks to the resistance of the discharge process! Please mount the 50W resistance to aluminum Chas was sold of the hot line to a metal plate to use! The circuit voltage would increase the precision of the input terminal (OVI would be absolutely O if you would like the theory to be understood you should check the over positioning theorem of electrical engineering). This circuit is a small voltage if the terminal contacts it so does not affect the actual calculation,
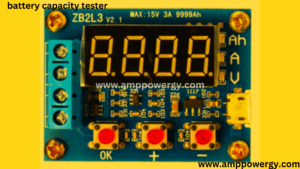
- Specifications
- Strength supply: DC4.5-6V (micro usb)
- Current operating: under 70mA
- 1.00V-1 5.00Vr 0.01V resolution. • Discharge voltage.’
- Voltage spectrum termination: 0.5-11.0V.
- New support overall resolution of 3.000k 0.001A
- Total calculating voltage error: 1%+0.02V.
- Current maximum error measurement: 1.5% ± 0.008A
- The maximal storage range for batteries is Ggg Ah (1 Ah = 1000mAh). If the value is less than IO Ah, XXXX is displayed to IO Ah or more, X.XXX will display, and so forth.
- Scale of board: 50 x 37mm
- Measurements: 50 x 37 x 17 mm (L x W x H, copper foot height included)
- Net weight of board: 28: (even columns)
- Weight of the resistor: roughly 27.
Working of the battery capacity tester
- You can totally charge the test battery.
- Attach a positive battery to the positive input, a negative input can be not reversed (the circuit can be destroyed by the reversing of load). Power on the test analyzer with micro IJS 3 power supply, connected to positive output and negative output (the laptop is LIS3 not available) and the battery voltage indicator.
- The “0K” button must only be pressed, the tester will automatically execute the required termination voltage with maximum charging voltage according to the battery and begins blinking three times before entering the test. If you need to use the manual termination voltage just press the “+” or the “-buttons” button, then after setting up, click the “0K” button and the “Ta Start Test” button, the termination Voltage Display starts with P.
- The test tester flips the electrostatic switch load on after beginning the test, the test data rotates from the release voltage (all the current release voltage (A to battery voltage, as battery voltage surpasses the termination voltage), the test load control shuts off the test tester.
- Error Codes
- Error codes and significance:
- Error 1: The voltage is greater than 15V.
- Error 2: The voltage of the battery is less than the end voltage.
- Error 3: The battery cannot charge discharge, or high resistance of circuit.
- Mistake 4 (current reaches 3.1 A): overcurrent
Implementation of prototype of battery tester
This section includes the way our concepts are implemented and the model we developed. The operational approach we discussed in the previous sections is the base.
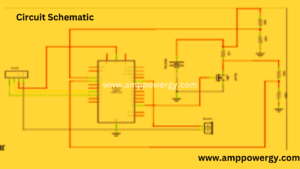
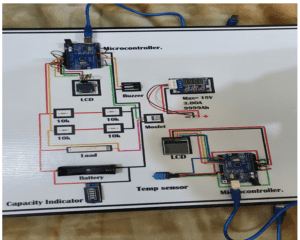
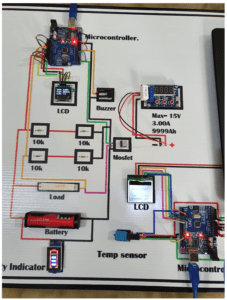
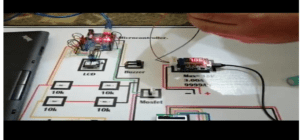
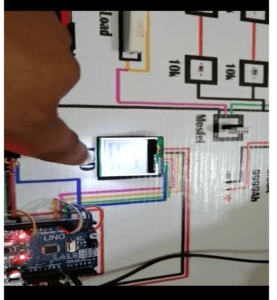
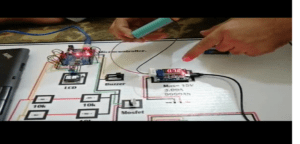
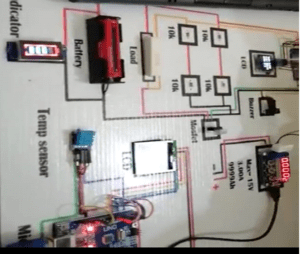
Circuit Diagram of the battery tester
Below is the schematic representation used to create the model which was also seen in the proposed project. This would be the key component or guideline for the whole system and clearly demonstrates the links and protocols in the project.
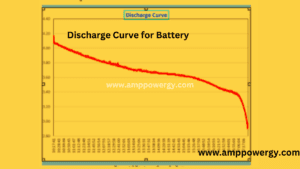
Discharge Curve of the battery tester
The following graph shows us the discharge curve of the battery. The initial voltage of the battery is 4.20 volts. When we put it on load the battery gradually discharged until it taken 6 hours to discharge.
Working Principle of the battery tester
If the battery is fine, send Arduino a directive to turn on the MOSFET, check the battery status. It allows current to flow through the capacitor from the anode end of the battery and completes the journey to the cathode end. This unloads the battery for a while. To discover the discharge current, Arduino tests the voltage around the charges and then separates them by the resistance. This was multiplied by time to get the value (capacity) of milliamp hour.
Voltage Measurement by battery tester
The voltage must be in the load resistor. Two voltage divider circuits are used to calculate the voltages. There are two resistors of 10k each. The divider output is connected to the Arduino A0 and A1 analog pins.
In our system the highest voltage is 4.2V for Arduino Analog Pin will measure up to 5V voltage. Then you may be curious why I excessively use two dividers. The trigger is that I will use the same multi-chemical battery tester in my future. This design can also be quickly modified to fulfill my objective.
Current Measurement by the battery tester
Current (I) = Voltage (V) — MOSFET/Resistance voltage decline
Note: Current suppose that the voltage drop is marginal around the MOSFET.
V = load resistor’s voltage and R = 10 Ohm.
The effect is in amplifications. To transform it into milli amperes multiply 1000.
Maximum current discharge = 4.2 / 10 = 420mA = 0.42A
Measurement of capacity:
Stored load = current x time (T). Stored load (Q).
The present has already been determined. In the latter calculation the only uncertainty is time. Arduino may use the Millis () method to calculate the time spent.
Prototype of the battery tester
Protype 1
Prototype 2
Prototype 3
Conclusion and Discussion on the battery tester
Results of the battery tester
Obtained outcome of this project is that we designed a battery testing device using a simple Arduino uno. The system works when voltage is detected through the MOSFET and the gate is opened. The voltage is discharged through voltage divider circuit as well as power resistor. The signal is sent to Arduino which in turn measures the voltage output and records the results. The results are then displayed on the Oled display attached to the system.
Capacity test by battery tester
To do the capacity test, first we have to select the test voltage of the battery which is in between 0 to 1 volt. Once selecting the test voltage, the tester will start to calculate the capacity of the battery and print it on the LCD which will take some time to discharge.
Result 1
Temperature and Humidity Test by the battery tester
This test is done to measure the humidity and temperature variation depending on the resistance of the thermistor to prevent the circuit from damage. To do this test we put a temperature sensor in the circuit.
Result 2
Charge Test by the battery tester
This test is done to check how much charge there is remaining in the battery. If the battery is charged, then the tester will go to test the test parameters of the battery while when the battery is empty then the tester will blow the indicator which means the battery needs] to be charged.
Result 3
5 Volts Battery Test by battery tester
Battery tester can test 5 volts battery as well and this test is done for 5 volts battery. When the battery is connected to the tester the voltage, current and capacity will be tested and can be seen on LCD.
Result 4
Battery indicator in a battery tester
This is the battery indicator of the battery. When we put the battery in its slot it, we show us the battery capacity through this indicator, and we can know how much capacity remains in the battery. When the charging quantity is lower than 25% it will only illuminate red outlines.
Result 5
- System Limitations
- Some of the most prominent limitations to 3d printing are as under:
- The system has separate devices for 5v and 12v battery.
- The system tends to display but cannot the actual temperature of the battery.
- The battery must be attached one at a time to the device.
- The battery display and temperature operate on different microcontrollers.
Future Work in battery tester
Big companies like automobile sector and electronic sector that have Hugh demand for batteries can make low-cost battery testers that provide fast and efficient results. This will help elongate the life of batteries as well as help consumers easily understand the real usage and time to change their battery. This if mass produced can solve many issues and can save people from disasters or problems before they happen.
Although battery analyzers are instruments to service batteries; battery control systems provide multi-purpose test functions for testing laboratories. Typical uses are life cycle testing and checking cell equilibrium in field imitation. Often such assessments may be automated by a particular device. Load capture requires load signatures to be processed for emulation of playback.
Many testing devices often control external loading units and atmospheric chambers. Performance checks and the checking of assurance statements are some applications of such schemes. A programmable power supply operated by a computer is the alternative to a battery testing device. Such a network allows stability but requires vigilant programming to escape tension and potential injury or fire in the case of an anomaly. A test battery like the Cadex C8000 offers safe charging and unloading programs which recognize the defective battery and safely detach a service. The device is overridden to run damaging experiments
Guidelines to Choosing a Battery Test System
⦁ Battery testers give only predictions, equivalent to a diagnostic examination or the weather forecast. None can do all this; a variety of approaches are necessary to achieve a complete evaluation.
- Most batteries retain a low internal resistance although their performance steadily declines with age.
- The resistance to batteries gives a snapshot only and cannot estimate end-of-life.
- Capacity is the primary predictor of fitness, but it is hard to quantify the measurement
- The battery needs several hours of normalization by charging or disembarking.
- To retain precision, coulomb counting requires periodic calibration.
- The maintenance of batteries avoids surprise defects and permits expected withdrawal.
- Battery monitoring on the premises includes troubleshooting for performance verification.
APPENDIX of the battery tester
Code of the Battery Tester Project:
#include i “U8glib.h”
#define i MOSFET_Pin i 2
#define i Bat_Pin i A0
#define i Res_Pin i A1
#define i Buzzer_Pin i 9
U8GLIB_SH1106_128X64 i u8g(U8G_I2C_OPT_NONE); i i // i I2C i / i TWI i i
float i Capacity i = i 0.0; i // i Capacity i in i mAh
float i Res_Value i = i 10.0; i i // i Resistor i Value i in i Ohm
float i Vcc i = i 4.64; i // i Voltage i of i Arduino i 5V i pin i
float i Current i = i 0.0; i // i Current i in i Amp
float i mA=0; i // i Current i in i mA
float i Bat_Volt i = i 0.0; i i // i Battery i Voltage i
float i Res_Volt i = i 0.0; i i // i Voltage i at i lower i end i of i the Resistor
float Bat_High = 4.3; // Battery High Voltage
float Bat_Low = 2.9; i // i Discharge i Cut i Off i Voltage
unsigned long previousMillis = 0; i // i Previous i time i in i ms
unsigned long millisPassed = 0; i i // i Current i time i in i ms
float sample1 =0;
float sample2= 0;
int x = 0;
int row = 0;
// i OLED i Display i Draw i Function i
void draw(void) {
u8g.setFont(u8g_font_fub14r); i // i select i font
if ( Bat_Volt <1) {
iu8g.setPrintPos(10,40); i i i i i i i i // i set i position
u8g.println(“No Battery!”);
}
else if ( Bat_Volt > Bat_High){
u8g.setPrintPos(25,40); // set i position
u8g.println(“High-V!”);
}
else if(Bat_Volt < Bat_Low){
u8g.setPrintPos(25,40);// i set i position
u8g.println(“Low-V!”); i
}
i else i if(Bat_Volt i >= i Bat_Low i && i Bat_Volt i < i Bat_High i i ){
i u8g.drawStr(0, i 20, i “Volt: i “); i i i // i put i string i of i display i at i position i X, i Y
u8g.drawStr(0, i 40, i “Curr: i “);
u8g.drawStr(0, i 60, i “mAh: i “);
i u8g.setPrintPos(58,20); i // i set i position
u8g.print( i Bat_Volt,2); i // i display i Battery i Voltage i in i Volt
i u8g.println(“V”); i
u8g.setPrintPos(58,40); i // i set i position
\u8g.print( i mA,0); i// i display i current i in i mA
u8g.println(“mA”); i
u8g.setPrintPos(58, i 60); i // i set i position
u8g.print( i Capacity i ,1); i // i display i capacity i in i mAh
}
}
// i Buzzer i Beep i Function i
i i void i beep(unsigned i char i delay_time){
i i analogWrite(9, i 20); i i i i i i // i PWM i signal i to i generate i beep i tone
i i delay(delay_time); i i i i i i i i i i // i wait i for i a i delayms i ms
i i analogWrite(Buzzer_Pin, i 0); i i // i 0 i turns i it i off
i i delay(delay_time); i i i i i i i i i i // i wait i for i a i delayms i ms i i
}
//Setup Function
void setup() {
Serial.begin(9600);
pinMode(MOSFET_Pin, OUTPUT);
pinMode(Buzzer_Pin, OUTPUT);
digitalWrite(MOSFET_Pin, LOW); // MOSFET is off during the start
Serial.println(“CLEARDATA”);
Serial.println(“LABEL,Time,Bat_Volt,capacity”);
//Serial.println(“Arduino Battery Capacity Tester v1.0”);
//Serial.println(“BattVolt Current mAh”);
}
//Main Loop Function
void loop() {
// Vcc = readVcc()/1000.0; // Conevrrt mV to Volt
// Voltage devider Out = Bat_Volt * R2/(R1+R2 ) // R1 =10K and R2 =10K
//************ Measuring Battery Voltage ***********
for(int i=0;i< 100;i++)
{
sample1=sample1+analogRead(Bat_Pin); //read the voltage from the divider circuit
delay (2);
}
sample1=sample1/100;
Bat_Volt = 2* sample1 *Vcc/ 1024.0;
// ********* Measuring Resistor Voltage ***********
for(int i=0;i< 100;i++)
{
sample2=sample2+analogRead(Res_Pin); //read the voltage from the divider circuit
delay (2);
}
sample2=sample2/100;
Res_Volt = 2* sample2 * Vcc/ 1024.0;
//********************* Checking the different conditions *************
if ( Bat_Volt > Bat_High){
digitalWrite(MOSFET_Pin, LOW); // Turned Off the MOSFET // No discharge
beep(200);
Serial.println( “Warning High-V! “);
delay(1000);
}
else if(Bat_Volt < Bat_Low){
digitalWrite(MOSFET_Pin, LOW);
beep(200);
Serial.println( “Warning Low-V! “);
delay(1000);
}
else if(Bat_Volt > Bat_Low && Bat_Volt < Bat_High ) { // Check if the battery voltage is within the safe limit
digitalWrite(MOSFET_Pin, HIGH);
millisPassed = millis() – previousMillis;
Current = (Bat_Volt – Res_Volt) / Res_Value;
mA = Current * 1000.0 ;
Capacity = Capacity + mA * (millisPassed / 3600000.0); // 1 Hour = 3600000ms
previousMillis = millis();
Serial.print(“DATA,TIME,”); Serial.print(Bat_Volt); Serial.print(“,”); Serial.println(Capacity);
row++;
x++;
delay(4000);
}
u8g.firstPage();
do {
draw ().
} while (u8g.nextPage() );
}
NOTE: REMOVE “I” FROM THE CODE TO COMPILE

Wow, fantastic weblog structure! How long have you been blogging for?
you made running a blog look easy. The full look of your
site is excellent, as smartly as the content!